Natriumhydrogencarbonat the right way to combine is an important talent for numerous functions, from baking to cleansing. This complete information will stroll you thru the method, overlaying completely different mixing strategies and their outcomes, together with mixing with different substances, and troubleshooting frequent points. We’ll additionally discover the chemical reactions and security precautions.
Understanding the properties of natriumhydrogencarbonat and the way it interacts with different elements is essential to reaching the specified outcomes. This information presents sensible recommendation and detailed directions, from light folding for baking to stirring for cleansing, offering insights into exact mixing ratios and anticipated outcomes.
Introduction to Sodium Bicarbonate (Baking Soda)
A humble white powder, sodium bicarbonate, or baking soda, whispers tales of culinary magic and past. This seemingly easy compound, an important ingredient in numerous recipes, possesses an enchanting array of properties that reach far past the kitchen. Its light effervescence and delicate alkalinity make it a flexible software in numerous industries, from medication to cleansing.This exceptional substance, with its seemingly benign nature, harbors each culinary and industrial functions.
Understanding its chemical composition, properties, and potential dangers permits us to understand its position in our each day lives and deal with it safely.
Chemical Formulation and Construction
Sodium bicarbonate, an important part in lots of chemical reactions, possesses a particular chemical construction. Its system, NaHCO 3, clearly identifies its constituent components: sodium (Na), hydrogen (H), carbon (C), and oxygen (O). This straightforward system masks an enchanting association of atoms, the place a sodium ion (Na +) is linked to a bicarbonate ion (HCO 3–). This construction provides rise to the distinctive properties of sodium bicarbonate.
Widespread Makes use of
Sodium bicarbonate finds widespread use throughout various fields. Its software in baking is well-known, offering the leavening motion that makes desserts and bread rise. Past the kitchen, it acts as a fireplace extinguisher part, a gentle antacid, and a cleansing agent. Its delicate alkalinity additionally makes it helpful in numerous industrial processes.
Types and Presentation
Sodium bicarbonate is often introduced in readily usable types. The most typical is a tremendous, white powder, simply measured and blended into recipes. For particular functions, resembling oral administration, sodium bicarbonate might also be obtainable in pill kind, providing a handy solution to devour it.
Security Precautions
Secure dealing with is paramount when working with sodium bicarbonate. All the time put on acceptable protecting gear, together with gloves, to forestall pores and skin irritation. Guarantee satisfactory air flow, particularly when dealing with bigger portions, to keep away from inhaling mud particles. Don’t combine sodium bicarbonate with robust acids, as this could produce probably hazardous fumes. Observe all directions rigorously when utilizing sodium bicarbonate in numerous functions.
Potential Well being Dangers
Improper dealing with or ingestion of sodium bicarbonate can pose sure well being dangers. Extended or extreme publicity to the powder can irritate the pores and skin or respiratory system. Ingestion of huge portions could result in digestive upset, resembling nausea or vomiting. It is essential to stick to really helpful dosage tips, notably when utilizing sodium bicarbonate as an antacid or for different medicinal functions.
All the time seek the advice of a healthcare skilled earlier than utilizing sodium bicarbonate for any well being situation.
Mixing Strategies for Totally different Purposes
Sodium bicarbonate, a flexible substance, finds functions far past baking. Its light nature permits for various mixing strategies, every essential to reaching the specified final result. Understanding these strategies unlocks the total potential of this exceptional compound, from creating fluffy desserts to successfully cleansing surfaces. From the fragile folding of dough to the vigorous stirring of a cleansing answer, the precise approach is paramount.
Mixing Strategies for Numerous Purposes, Natriumhydrogencarbonat the right way to combine
Totally different functions necessitate various mixing approaches. The gentleness of the substance permits for a variety of methods, every affecting the ultimate consequence. Cautious consideration of the meant final result and the character of the elements is crucial. A delicate fold is acceptable for baking, whereas a extra strong stirring motion is appropriate for cleansing. This part delves into the nuances of blending sodium bicarbonate in numerous contexts.
| Software Kind | Mixing Methodology | Anticipated End result | Security Issues |
|---|---|---|---|
| Baking | Light Folding | Mild and ethereal texture, optimum rise, and even distribution of elements. This method prevents the incorporation of extra air, which may result in a tricky or dense ultimate product. | Keep away from overmixing, because it develops gluten, probably leading to a tricky or chewy texture. Light folding is essential to reaching a lightweight and fluffy consistency. |
| Cleansing | Stirring | Efficient cleansing motion, dissolving sodium bicarbonate into water, making a potent cleansing answer. The agitation ensures even distribution of the cleansing agent. | Use in a well-ventilated space to keep away from inhaling any mud or fumes. Keep away from direct contact with eyes or delicate pores and skin. |
| Making a Paste | Mixing with Water (or different liquids) | A easy, constant paste best for making use of to surfaces. The combination shouldn’t be too thick or too skinny. | Make sure the ratio of sodium bicarbonate to liquid is acceptable. Extreme liquid could dilute the paste, whereas inadequate liquid can create a lumpy or uneven texture. |
Mixing Ratios for Baking Recipes
Exact mixing ratios are vital in baking, impacting the ultimate product’s texture and style. The correct proportion of elements straight influences the result. A slight deviation from the really helpful ratios can lead to a noticeably completely different ultimate product.
| Recipe | Sodium Bicarbonate (grams) | Different Substances (grams) | Anticipated End result |
|---|---|---|---|
| Fundamental Baking Soda Biscuits | 2 grams | 100 grams all-purpose flour, 50 grams chilly butter, 30 grams ice water | Tender, flaky biscuits with a barely ethereal texture. |
| Fluffy Pancakes | 1 gram | 100 grams all-purpose flour, 100 ml milk, 1 egg, 10 grams sugar | Mild and ethereal pancakes, with a great rise and tender crumb. |
| Fast Bread | 3 grams | 100 grams all-purpose flour, 50 grams sugar, 100 ml milk, 1 egg, 10 grams melted butter | A young and barely moist fast bread with a pleasing aroma. |
Significance of Exact Mixing Ratios
Correct measurements and constant mixing are important in reaching the specified final result in baking. The interaction of elements, notably the interplay between sodium bicarbonate and acidic elements, is a vital issue. Small variations in ratios can have an effect on the ultimate product’s rise, texture, and style. A slight adjustment can dramatically alter the top consequence.
Mixing with Different Substances
A symphony of reactions unfolds when sodium bicarbonate, the standard baking soda, encounters different substances. Its effervescent nature, a fascinating dance of bubbles and fizzing, is a testomony to its chemical interactions. This exploration delves into the fascinating chemistry that happens when baking soda meets acids and numerous liquids, unveiling the fascinating transformations that ensue.
Reactions with Acidic Substances
Sodium bicarbonate, with its inherent alkalinity, readily engages in chemical reactions with acidic substances. This interplay ends in the manufacturing of carbon dioxide gasoline, an important part in quite a few functions, from baking to cleansing. The discharge of carbon dioxide is accompanied by the formation of a salt and water, a traditional acid-base neutralization response.
Sodium bicarbonate + Acid → Carbon dioxide + Water + Salt
This response is a elementary precept in understanding the chemistry behind baking, effervescent drinks, and numerous cleansing functions.
Examples of Reactions
Quite a few examples showcase the dynamism of sodium bicarbonate’s interactions with acids. A easy instance includes mixing sodium bicarbonate with vinegar, a typical family acid. The fast launch of carbon dioxide gasoline creates a dramatic fizzing impact, producing a visually partaking demonstration of chemical transformation. One other illustrative response includes mixing sodium bicarbonate with lemon juice, additionally an acidic substance.
The result’s the same effervescent response, highlighting the universality of this chemical interplay. The depth of the response is dependent upon the focus of each the acid and the sodium bicarbonate.
Reactions with Totally different Liquids
The interplay of sodium bicarbonate with numerous liquids, past acidic options, reveals additional fascinating traits. Mixing sodium bicarbonate with water produces a comparatively calm and impartial combination. The sodium bicarbonate dissolves readily in water, exhibiting a notable distinction in habits in comparison with its response with acids. Including sodium bicarbonate to exploit creates a suspension, the place the baking soda particles are dispersed all through the liquid.
The ensuing combination is comparatively steady, showcasing a unique response in comparison with its interplay with acidic substances.
Results of Mixing
The results of blending sodium bicarbonate with completely different substances differ considerably. Mixing with acidic substances, like vinegar or lemon juice, results in a vigorous effervescence, accompanied by the manufacturing of carbon dioxide gasoline, a visual signal of the chemical response. The combination turns into noticeably hotter, a testomony to the power launched in the course of the chemical transformation. The depth of the fizzing and the diploma of temperature change rely on the focus of the acid and the sodium bicarbonate.
The combination with water, in distinction, exhibits a comparatively delicate response, with the baking soda dissolving into the liquid with out noticeable effervescence. The combination with milk demonstrates a unique response sample, with the baking soda particles suspended within the liquid, showcasing a notable visible distinction in comparison with its interactions with acids or water.
Security Precautions
When dealing with sodium bicarbonate and mixing it with different substances, security precautions are paramount. All the time work in a well-ventilated space to keep away from inhaling any launched gases. Keep away from contact with eyes and pores and skin to forestall irritation or potential burns. Put on acceptable security gear, resembling gloves and goggles, particularly when coping with concentrated options or bigger portions of reactants.
By no means combine sodium bicarbonate with robust oxidizing brokers, as such mixtures can result in probably harmful reactions. Observe secure laboratory practices and seek the advice of security tips every time needed to make sure a secure and profitable mixing course of.
Mixing for Particular Outcomes
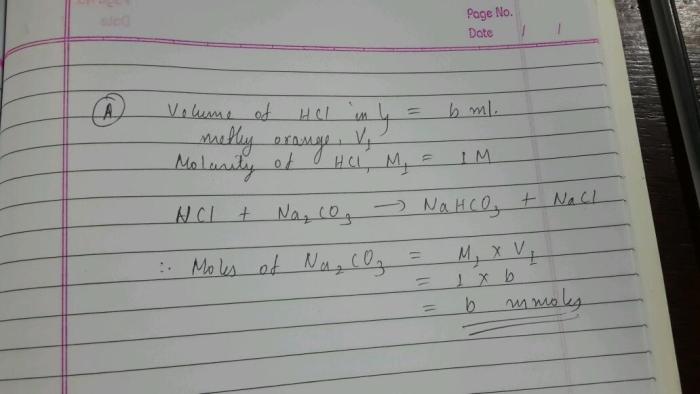
Sodium bicarbonate, a humble white powder, transforms into a flexible ally when blended with the precise elements. Its delicate effervescence, a whisper of magic, might be harnessed for numerous duties, from soothing culinary creations to tackling cussed grime. This exploration delves into the nuanced artwork of blending sodium bicarbonate to realize particular outcomes, from easy pastes to potent cleansing options.The important thing to profitable mixing lies in understanding the response of sodium bicarbonate with different substances.
Its alkaline nature, mixed with its means to launch carbon dioxide, permits for a variety of functions. Cautious consideration of proportions and mixing methods unlocks the total potential of this exceptional compound.
Making a Paste
A paste, a easy, thick combination, is commonly desired for its software properties. To attain this consistency with sodium bicarbonate, the bottom line is to include a liquid medium, resembling water, whereas progressively including the bicarbonate. The addition of a thickening agent, like cornstarch or arrowroot powder, additional enhances the paste’s texture and prevents it from changing into too runny.
This technique is invaluable in creating poultices for minor pores and skin irritations or as a thickener for sure recipes.
Making a Suspension
A suspension, in contrast to a paste, includes dispersing stable particles in a liquid with out dissolving them. Sodium bicarbonate can be utilized in suspensions for numerous cleansing functions, the place the abrasive motion of the particles is helpful. The hot button is to combine the sodium bicarbonate with a liquid, guaranteeing the particles are evenly distributed. A delicate stirring technique is commonly most well-liked to keep away from clumping or settling.
Cleansing Purposes
Sodium bicarbonate’s delicate abrasiveness and alkaline properties make it a superb cleansing agent for numerous surfaces. Mixing it with water or vinegar creates a robust but secure cleansing answer for counter tops, sinks, and even ovens. For harder stains, a paste of sodium bicarbonate and water might be utilized and left to work its magic. The effectiveness of sodium bicarbonate in cleansing is amplified when mixed with different cleansing brokers.
Baking Recipes
Sodium bicarbonate performs an important position in baking, performing as a leavening agent that produces carbon dioxide gasoline, making baked items rise. Mixing strategies differ relying on the kind of baked good. Fast breads, resembling muffins and scones, typically profit from a dry-wet technique, the place dry elements are mixed individually from moist elements, after which mixed gently.
For cookies and desserts, a creaming technique is commonly used, the place fat and sugars are creamed collectively earlier than including the dry elements, guaranteeing the fragile texture of the ultimate product.
- Fast Breads: Mix dry elements (flour, sugar, baking powder) in a single bowl and moist elements (milk, eggs, oil) in one other. Gently fold the moist elements into the dry till simply mixed. Overmixing can result in a tricky product.
- Cookies: Cream collectively butter and sugar till mild and fluffy. Step by step add eggs and blend effectively. Incorporate dry elements (flour, baking soda, salt) separately, mixing gently till simply mixed.
- Muffins: Cream collectively butter and sugar till mild and fluffy. Beat in eggs separately, then alternate including dry elements (flour, baking powder, salt) and milk, mixing till simply mixed. This technique ensures a lightweight and ethereal texture.
Cleansing Options
Mixing sodium bicarbonate with different elements creates tailor-made cleansing options. An answer of sodium bicarbonate and vinegar, as an example, is a potent deodorizer and cleaner for numerous surfaces. One other efficient cleansing answer is a mix of sodium bicarbonate and lemon juice, which is very efficient for brightening and deodorizing. The flexibility of sodium bicarbonate extends to cleansing options, creating a variety of potent but secure cleansing choices.
| Cleansing Job | Cleansing Answer |
|---|---|
| Deodorizing | Sodium bicarbonate + vinegar |
| Brightening | Sodium bicarbonate + lemon juice |
| Common Cleansing | Sodium bicarbonate + water |
Troubleshooting Widespread Points
Mixing sodium bicarbonate (baking soda) generally is a pleasant expertise, reworking easy elements into one thing extraordinary. Nonetheless, like all course of, occasional challenges can come up. Understanding these potential pitfalls and their options empowers you to confidently navigate the blending course of and obtain the specified outcomes each time. From pesky clumps to uneven mixtures, this part equips you with the information to troubleshoot and conquer any mixing mishap.Troubleshooting is essential for reaching constant and passable ends in any mixing course of.
By figuring out the basis causes of issues and implementing efficient options, you possibly can make sure the success of your baking endeavors. A eager understanding of those points lets you refine your approach, and in the end, obtain the proper mix every time.
Figuring out and Addressing Clumping
Clumping is a typical frustration, disrupting the graceful texture you want. Understanding the causes is essential to stopping these unwelcome aggregates. Moisture, improper storage, and even variations within the sodium bicarbonate itself can contribute to clumping.
- Moisture Content material: Extra moisture within the baking soda could cause it to clump collectively. Make sure the sodium bicarbonate is saved in a cool, dry place, and ideally in an hermetic container to forestall moisture absorption. If moisture is suspected, gently dry the baking soda in a low oven (round 100°C) for a brief interval, or use a meals dehydrator.
This course of might be useful in case your baking soda has been uncovered to excessive humidity or moisture.
- Improper Storage: Improper storage, resembling leaving the container open or in a moist surroundings, permits moisture to penetrate and trigger clumping. Use hermetic containers to take care of the integrity of the baking soda’s texture. Common inspection of your baking soda for indicators of clumping is important for guaranteeing optimum high quality.
- High quality of Baking Soda: Often, the standard of the baking soda itself could also be compromised. If clumping persists regardless of correct storage and dealing with, think about buying a recent batch of baking soda.
Troubleshooting Uneven Mixtures
Uneven mixtures can result in inconsistencies in texture and look. Understanding the components contributing to this concern is essential for reaching a uniform final result. Inconsistent mixing methods or differing particle sizes within the elements can result in this drawback.
- Inconsistent Mixing Strategies: Guarantee thorough mixing, utilizing a whisk, spatula, or electrical mixer, relying on the appliance. Step by step incorporate elements to keep away from creating pockets of undissolved particles. This thoroughness is essential to reaching a uniform consistency.
- Ingredient Particle Measurement Variation: If the elements you might be mixing have vastly completely different particle sizes, this could result in an uneven distribution. Utilizing a sieve or blender might help guarantee a extra uniform particle measurement, leading to a smoother combination.
Addressing Inadequate Response
An inadequate response can result in undesirable outcomes in recipes. A number of components can contribute to this, and understanding these causes can result in efficient options. Variations within the quantity of an ingredient, or the inaccurate temperature of the combination, can have an effect on the chemical response, impacting the specified outcomes.
- Incorrect Measurement: Exact measurement of all elements is important for a profitable response. Utilizing correct measuring instruments and following the recipe’s directions rigorously minimizes errors in measurement, guaranteeing a exact response.
- Temperature Variations: Temperature can affect the pace and effectivity of chemical reactions. Sustaining a constant temperature all through the blending course of might help obtain the specified final result. Adjusting the temperature can considerably affect the result of the response. In some instances, warming the elements barely can speed up the response.
Illustrative Examples
A profound understanding of sodium bicarbonate (baking soda) arises not simply from its chemical composition however from its sensible software. Think about the satisfying fizz of a home made volcano, the light rise of a fluffy cake, or the refined tang of a superbly balanced dish. Every of those experiences hinges on the exact mixing of baking soda, a humble ingredient with a shocking versatility.
Mastering the artwork of blending sodium bicarbonate opens doorways to culinary creations and scientific explorations.This part delves into the sensible software of sodium bicarbonate, showcasing the meticulous steps concerned in its use throughout various eventualities. We’ll visualize the transformation of sodium bicarbonate, highlighting its interplay with numerous substances and demonstrating how the right mixing approach can result in fascinating outcomes.
Mixing Sodium Bicarbonate for a Fluffy Cake
Mastering the artwork of blending baking soda for a fluffy cake includes a fragile dance between elements. Fastidiously measuring and mixing elements is essential to reaching the specified texture and taste. The picture beneath exhibits the preliminary stage, with dry elements meticulously measured and positioned in a bowl.
Picture Description: A transparent glass bowl holds sifted flour, granulated sugar, baking soda, and a pinch of salt. A wood spoon rests on the aspect of the bowl, hinting on the subsequent steps within the mixing course of. The lighting is brilliant and even, highlighting the feel and readability of the elements.
Picture Description: The following picture exhibits the moist elements being progressively included into the dry elements. A whisk is getting used to mix the egg yolks, milk, and melted butter with the dry elements in a easy movement. The bowl is positioned in a approach that exhibits the combination thickening because the whisk incorporates the moist elements into the dry. The lighting remains to be brilliant and clear, highlighting the consistency of the combination.
Picture Description: A ultimate picture captures the batter after thorough mixing. The batter is mild, ethereal, and easy, freed from lumps. The consistency is essential for a profitable cake. A spatula is seen, prepared for the following stage of preparation, pouring the batter into the cake pan. The lighting is brilliant and even, permitting for clear visualization of the batter’s texture.
Mixing Sodium Bicarbonate for a Baking Soda Volcano
The dramatic response between sodium bicarbonate and an acid, like vinegar, creates a mesmerizing visible spectacle. The cautious addition of acid to sodium bicarbonate triggers a fast launch of carbon dioxide gasoline, producing a miniature volcanic eruption.
Picture Description: The primary picture exhibits a small, clear plastic cup crammed with baking soda. A second, smaller cup, containing white vinegar, is positioned subsequent to the baking soda cup. Each are clearly seen. The scene is about for the response.
Picture Description: The following picture captures the second when the vinegar is rigorously poured into the baking soda. The combination begins to bubble vigorously, making a effervescent motion that slowly rises within the baking soda.
Picture Description: The ultimate picture depicts the end result of the response, showcasing a small-scale volcanic eruption. The carbon dioxide gasoline, quickly increasing, pushes the combination upward, resembling a miniature volcano. The dramatic launch of gasoline and the ensuing foam are clearly seen.
Visible Information to Mixing Sodium Bicarbonate in Recipes
This information will present step-by-step directions for mixing sodium bicarbonate in numerous recipes.
- Measuring: Precisely measure the required quantity of sodium bicarbonate utilizing a measuring spoon or scale. This exact measurement is essential for reaching the specified final result within the ultimate product.
- Combining: Mix the sodium bicarbonate with different dry elements (like flour or sugar) in a bowl. This ensures even distribution all through the recipe.
- Including Liquids: Step by step add liquids (like milk or water) to the combination, whisking or stirring constantly. This helps forestall lumps and ensures even mixing.
- Mixing: Combine the elements totally till a easy, lump-free consistency is achieved. The right mixing approach ensures the specified final result, from fluffy desserts to tender biscuits.
Chemical Response of Sodium Bicarbonate with Acids
The response between sodium bicarbonate and acids, like vinegar or lemon juice, is a traditional instance of a chemical response. The interplay produces carbon dioxide gasoline, an important part in baking and different functions.
NaHCO3(s) + H +(aq) → CO 2(g) + H 2O(l) + Na +(aq)
Picture Description: The picture depicts a chemical response, displaying a beaker containing sodium bicarbonate. A transparent liquid, representing an acid, is slowly being poured into the beaker. The response is indicated by the formation of bubbles, that are carbon dioxide gasoline, that rise from the combination. The response is visually putting and demonstrates the interplay between sodium bicarbonate and acids.
Mixing Ratios and Proportions: Natriumhydrogencarbonat How To Combine
A symphony of textures and flavors awaits once you grasp the artwork of blending sodium bicarbonate. This seemingly easy ingredient, typically simply baking soda, holds a shocking versatility, reworking from leavening agent to cleansing answer, relying on the ratios you use. Understanding these ratios unlocks a world of culinary potentialities and sensible functions, permitting you to fine-tune your creations with precision.
Baking Recipes and Mixing Ratios
Baking soda’s magic lies in its means to react with acidic elements, producing carbon dioxide gasoline that makes baked items rise. The exact steadiness between baking soda and acid is vital for reaching the specified texture and quantity. Totally different recipes demand completely different proportions, influenced by the kind and quantity of acid current.
- Fast Breads: For fast breads like muffins and pancakes, the ratio of baking soda to acid is often decrease than in desserts or cookies. The acid content material within the moist elements typically comes from buttermilk, lemon juice, or vinegar. This decrease ratio ensures a young, mild texture with out over-developing the batter.
- Muffins: Cake recipes typically make the most of a better proportion of baking soda and acid, guaranteeing a constantly fluffy and ethereal cake. The acid within the cake batter, usually from buttermilk or lemon zest, interacts with the baking soda to create a extra pronounced leavening impact. The exact ratio is essential for the specified top and construction.
- Cookies: Cookie recipes typically rely much less on the direct interplay of baking soda and acid for leavening. As a substitute, they typically make the most of different leavening brokers like baking powder. Nonetheless, small quantities of baking soda could also be utilized in mixture with different leavening brokers or acidic elements, resembling cocoa powder, to reinforce texture. The ratio is rigorously adjusted to provide a fascinating texture and stop over-rising.
Adjusting Mixing Ratios for Desired Outcomes
Wonderful-tuning the blending ratios lets you tailor the ultimate product to your particular preferences. For instance, a barely increased ratio of baking soda to acid would possibly end in a extra substantial rise, but additionally probably a barely extra bitter style. Conversely, a decrease ratio can create a softer, extra tender final result. The specified final result will rely on the precise recipe and desired taste profile.
| Recipe | Baking Soda (grams) | Acid (grams) | Notes |
|---|---|---|---|
| Fundamental Biscuits | 10 | 15 | Use buttermilk for the acid. |
| Chocolate Chip Cookies | 5 | 8 | Mix with baking powder for a greater rise. |
| Fluffy Pancakes | 12 | 18 | Use a mix of lemon juice and buttermilk for a tangy taste. |
| Vanilla Cupcakes | 15 | 20 | Make sure the acid supply is well-mixed into the batter. |
Experimentation is essential. Begin with the really helpful ratios, then progressively alter to realize your most well-liked final result. Slightly apply will will let you intuitively perceive how completely different ratios have an effect on the ultimate product.
Illustrative Examples
Think about a state of affairs the place you make biscuits. Utilizing a decrease ratio of baking soda to buttermilk would possibly yield a denser, much less ethereal biscuit. Alternatively, a better ratio would possibly end in a too-light and crumbly biscuit. Discovering the proper steadiness via experimentation will refine your baking expertise and result in scrumptious outcomes.
Remaining Assessment

In conclusion, mixing natriumhydrogencarbonat successfully is dependent upon understanding its properties and the way it interacts with different substances. This information supplies an in depth strategy to mixing, from completely different strategies for numerous functions to troubleshooting frequent points. By following the supplied tips, you possibly can obtain the specified final result in baking, cleansing, and different functions involving natriumhydrogencarbonat.
Solutions to Widespread Questions
How a lot sodium bicarbonate ought to I take advantage of for a cake?
The quantity of sodium bicarbonate wanted for a cake recipe varies. Seek advice from the precise recipe for the right mixing ratio.
What occurs when sodium bicarbonate mixes with vinegar?
The combination fizzes and produces carbon dioxide gasoline on account of a chemical response between the sodium bicarbonate and the acidic vinegar.
Why are there clumps in my sodium bicarbonate combination?
Clumps would possibly happen if the sodium bicarbonate is just not correctly dispersed or if it is not dry sufficient. Make sure the powder is totally dry and use a whisk or a sifter for even mixing.
What security precautions ought to I take when mixing sodium bicarbonate with different substances?
All the time work in a well-ventilated space and keep away from inhaling any fumes. Put on gloves and security goggles when dealing with the combination, particularly if working with acids.

